metallic bond examples
But here are explanations of metallic bonding in some metals ie aluminium magnesium and sodium. Metal elements can undergo destruction.
 |
| Chemical Bonding Definition Types Properties Examples |
Generally all metals are metallic bond examples.
. Sodium Aluminium Magnesium Copper Iron In one of the geometrical arrangements like body central cubic arrangement hexagonal close-packed or. And the positive sodium atom immersed into. A series of free IGCSE Chemistry Activities and Experiments Cambridge IGCSE Chemistry. Examples of how to use metallic bond in a sentence from the Cambridge Dictionary Labs.
Magnesium has 2 valence electrons which are in the 3s energy level. These two elements are metals and their outermost electrons are loosely bound to their nuclei which forms a group of electrons that can easily be moved from one element to another. Examples of Metallic Bonding Alkali metals like sodium and potassium Alkaline earth metals like calcium and magnesium Transition metals like iron titanium gold tungsten. Furthermore we also learned that these bonds are non-directional.
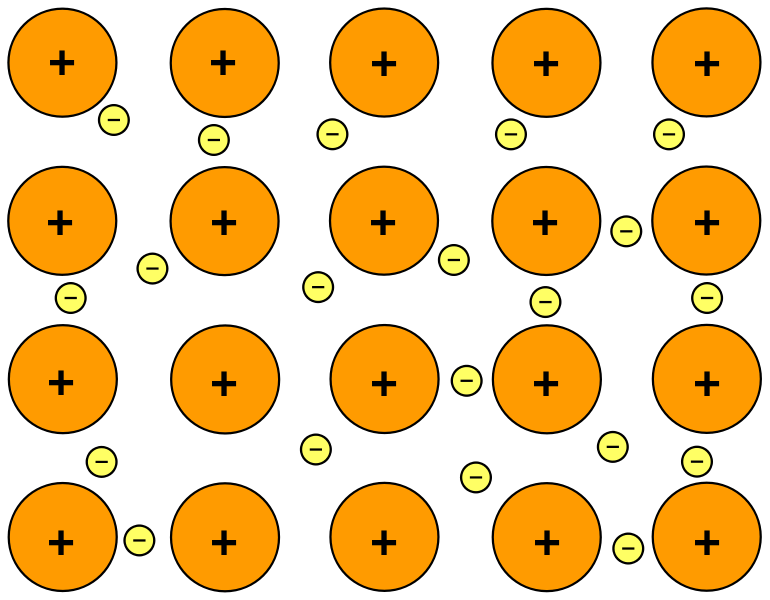
Metallic bonds are seen in pure metals and alloys and some metalloids. The electrostatic force of attraction between kernels and mobile delocalised electrons are the metallic bonds. The electrons that are in the outermost orbit of an atom are called valence electrons. Orbitals are the spatial representations in which the electrons are located with a.
Bonds between silver Ag atoms. Metallic Bond Examples Some examples of metallic bonds include magnesium sodium and aluminum. In metallic bonding the valence electrons are not localized to any one particle. Example Sodium has one valence electron.
Metals such as silver iron gold aluminium are bonded by metallic bonds via delocalised electrons. What is metallic bonding example. Bonds between gold Au. The word kernel is used to represent the.
Other sorts of chemical. Remember that a metal bond is simply the electrostatic attraction between positive metal ions and the sea of delocalised electrons so this creates stronger bonding. Like covalent bonds metallic bonds form between two atoms with similar electronegativity values. For example graphene an allotrope of carbon exhibits two-dimensional metallic bonding.
Metallic bonds are extremely common in the atomic world of metals so any pure metallic element is a possible example. This electron can delocalize through out the metal crystal to form the metallic bond. Metallic bond examples are. Examples of a metallic bond include gold and copper.
The free electrons act as a bond between the ions with a positive charge. Metallic bonds can be found in pure metals and alloys as well as certain metalloids. Atoms that form metallic bonds are. For example graphene a carbon allotrope has two-dimensional metallic bonding.
 |
| Metallic Bond Definition Factors And Explanation |
 |
| Metallic Bond Teaching Resources Teachers Pay Teachers |
 |
| Difference Between Ionic Covalent And Metallic Bonds Definition Formation Properties |
 |
| Ionic Bonding Examples 1 3 5 Cie A Level Chemistry Revision Notes 2022 Save My Exams |
 |
| Chemical Bonding Part 6 Metallic Bonding Youtube |
Posting Komentar untuk "metallic bond examples"